Medical Startups Have Zero Tolerance for Error — Precision Components Are Non-Negotiable
No failures. No delays. Just modelling, samples, and on-time delivery.
If you’re building a medical device startup, you don’t get the luxury of learning through trial and error. One overlooked component issue can quietly erase months of progress—sometimes right before a regulatory review, pilot build, or investor milestone.
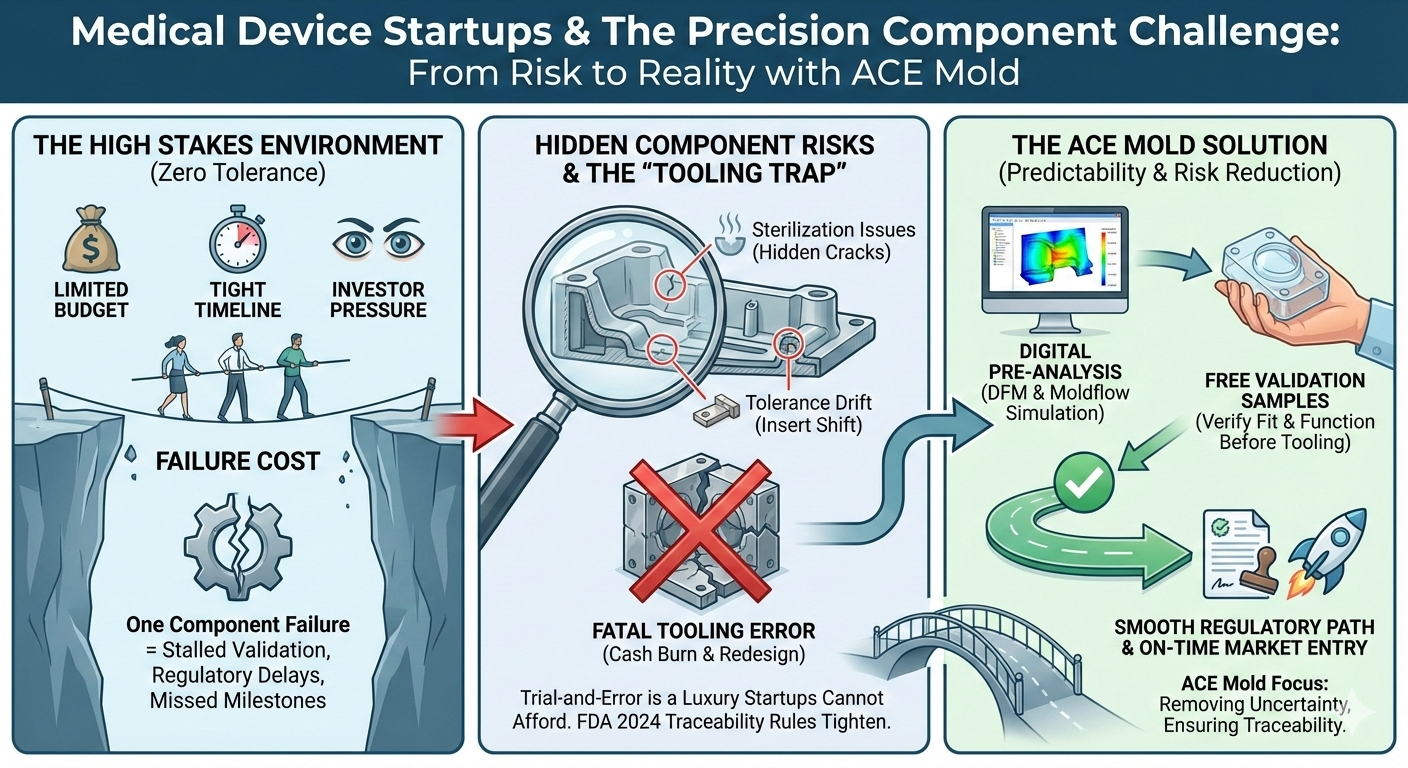
Early failures rarely come from the core technology. More often, they originate in supporting components: a casing that distorts, an insert that shifts during molding, or a bearing housing that loses tolerance after sterilization. These problems are subtle, difficult to trace, and extremely costly to correct once tooling is complete.
With the medical device plastics market growing at 7.4% per year and the FDA tightening traceability expectations in 2024, startups are under more pressure than ever to make the right component decisions from the very beginning.
When One Component Decides the Fate of a Startup
In medical device startup manufacturing, a single precision component can determine whether a product moves forward—or stalls indefinitely.
A small deviation in:
- precision molded bearing housings
- medical device casing molding
- an insert-molded interface
- or a circular structural feature
can trigger failed validation builds, repeated testing cycles, or regulatory questions that delay market entry.
This is why medical precision components are not just production inputs. They directly influence timelines, funding confidence, and regulatory outcomes.
Why Medical Startups Feel the Risk More Intensely
Unlike established OEMs, startups operate with lean teams and limited safety nets. Procurement, R&D, validation, and documentation often fall on the same few people.
When a supplier underperforms, the impact escalates quickly:
- rework after tooling
- stalled PPAP or validation cycles
- delayed submissions
- missed investor milestones
As a result, startups don’t just look for parts. They look for clarity, predictability, and risk reduction.
At this stage, many designs are still evolving. What looks acceptable in early CAD or bench testing can behave very differently once exposed to real molding conditions, assembly forces, and sterilization cycles. Wall thicknesses get adjusted, materials change, inserts are repositioned, and tolerances tighten as the device moves closer to validation. This evolution is normal—but it also means trial-and-error manufacturing is a luxury startups cannot afford.
Zero Tolerance Isn’t a Slogan — It’s an Operating Reality
Every medical device contains multiple precision-critical elements:
- precision molded medical housings
- insert-molded features
- bearing interfaces
- alignment- or load-critical structures
These components must remain stable through assembly, real-world use, and sterilization. That makes sterilization-safe plastic components and dimensional stability in molded parts essential—not optional.
A casing that passes visual inspection may still crack after autoclaving.
A bearing seat slightly out of spec can introduce vibration or performance drift.
An insert shift too small to see can disrupt automated assembly.
The challenge isn’t visibility. It’s predictability.
Why Medical Startups Actively Search for Precision Suppliers
Medical startups actively seek precision suppliers because they have no room for iteration after tooling.
Teams developing:
- diagnostic devices
- surgical tools
- wearables
- home-care equipment
must lock in reliable components before clinical trials or FDA submissions. That’s why they search for:
- an insert molding supplier medical teams can trust
- partners focused on injection molding failure prevention, not just production output
Tooling Risk: The One Mistake Startups Can’t Absorb
Tooling errors are manageable for large OEMs. For startups, they’re often fatal.
A single mistake can mean:
- redesigning molds
- reworking cooling or gating
- resetting validation timelines
- burning cash with no forward progress
This is why startups increasingly insist on DFM and Moldflow simulation before steel is cut. They want problems exposed digitally long before they become physical.
Why Process Transparency Matters More Than Cost
Under the FDA’s updated 2024 expectations, regulators now evaluate not only how a component performs but also how it was made.
A warped housing or unstable insert can lead to:
- documentation gaps
- failed verification steps
- delayed 510(k) pathways
- repeated sterilization testing
For startups, these aren’t technical inconveniences. They are regulatory bottlenecks because traceability is no longer a “nice to have.” It’s becoming the baseline expectation.
Where ACE Mold Changes the Equation
Many suppliers can mold parts. ACE Mold focuses on removing uncertainty.
By combining DFM and Moldflow simulation, ACE Mold identifies failure risks early timely addressing warpage, shrinkage, insert movement, and tolerance drift before tooling begins. This stabilizes:
- precision molded medical housings
- precision molded bearing housings
- insert-molded geometries
- repeatability across builds
To further reduce startup risk, ACE Mold provides free validation samples, allowing teams to confirm fit, function, and stability before committing to production.
And with dependable, on-time delivery, startups can protect validation schedules and regulatory timelines.
No failures. No delays. Just modelling, samples, and on-time delivery.
Why This Matters Now
Medical startups operate under:
- strict regulatory oversight
- limited budgets
- aggressive timelines
- high investor scrutiny
They don’t need a vendor who simply supplies parts. They need a partner who brings engineering clarity, traceability awareness, and failure prevention into the process.
That’s where ACE Mold fits.
Why Free Validation Samples Matter to Medical Startups
For medical startups, free validation samples aren’t a perk rather a necessity..
With tight budgets and compressed timelines, teams need to verify fit, function, dimensional stability, and sterilization performance before committing to full production. ACE Mold treats sampling as part of an engineering-led process, not a sales gesture.
Through DFM and Moldflow simulation, potential issues are addressed upfront, then validated with real samples that teams can test, measure, and document with confidence.This is what defines a premium precision supplier: removing uncertainty early and preventing costly mistakes later.
If your team needs precision components that perform as expected from the very first sample, ACE Mold is ready to support your next stage quietly, reliably, and without surprises.





